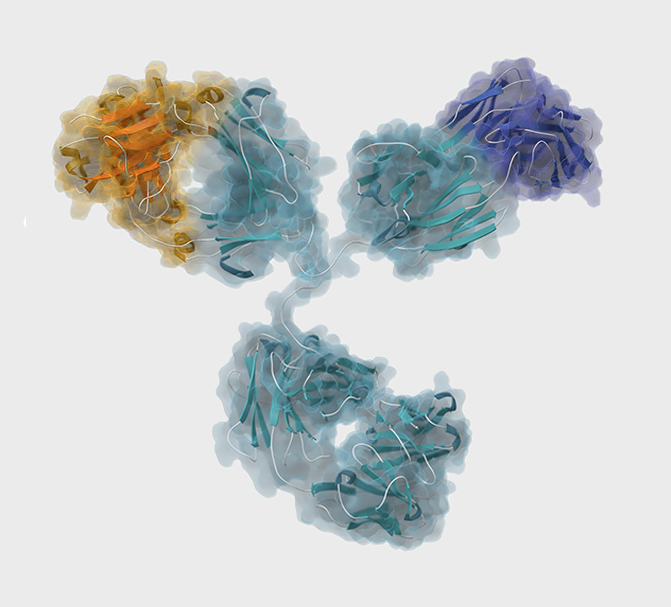
Harnessing the Full Potential of Therapeutics With Site-Directed Precise Medicines
Our discovery that oxMIF is generated exclusively in the oxidative environment at sites of inflammation provides the basis for a groundbreaking and versatile approach to direct therapeutics exclusively to diseased sites.
Candidate
Entity & Indication
Target
| Discovery
| Pre-clinical
| Phase 1
Immunology
Oncology
ON104
ON-08
ON203
ON102
Therapeutic mAb
RA, Nephritis, IBD & Asthma
Therapeutic bispecific mAb
Alzheimer‘s & MS
Therapeutic mAb
Colorectal, Lung Cancer
Radioimmunoconjugate diagnostic
Cancer & Inflammation
oxMIF
oxMIF
oxMIF
oxMIF
Immunology: ON104
Pre-clinical
Immunology: ON-08
Discovery
Oncology: ON203
Pre-clinical
Oncology: ON102
Pre-clinical
Current candidates:
ON104 is a second-generation monoclonal anti-oxMIF antibody with abolished effector functions, tailored to the therapeutic needs of patients living with chronic inflammatory and autoimmune disorders. ON104 neutralizes the pleiotropic disease promoting activities of oxMIF. In particular, as oxMIF can override the immunosuppressive effects of glucocorticoids, ON104 synergizes with current immunosuppressant therapeutics.
Currently, OncoOne is advancing ON104 through late-stage pre-clinical development for indications like Rheumatoid Arthritis (RA), Nephritis, Inflammatory Bowel Disease (IBD), and Asthma. Optimized bio-physiochemical properties of ON104 support a high-dose formulation so that patients can perform self-injections at home using an autoinjector pen.

The development of neuroprotective drugs is a significant gap in medical care, as these diseases progressively impact patients' quality of life and ability to perform daily activities. OncoOne is dedicated to addressing these challenges through its proprietary selective targeting approach. By leveraging the presence and pathological activities of oxMIF at sites of chronic inflammation, OncoOne aims to neutralize its disease-promoting activity using bispecific antibodies capable of crossing the blood-brain barrier.
Currently, ON-08 is in the initial discovery stage.
ON203 is a second-generation monoclonal anti-oxMIF antibody designed to address the specific therapeutic requirements of patients with solid tumors. This antibody has been optimized for enhanced tumor penetration and improved pharmacokinetic properties. ON203 effectively inhibits oxMIF-mediated pro-tumorigenic functions and demonstrates cytotoxic functions by inducing antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), offering significant therapeutic potential in solid tumors. Its mechanism of action allows for its use either as a standalone treatment or in combination with standard-of-care therapies.
ON203 is ready for filing IND/IMPD and to address the unmet medical needs of patients facing solid tumors of the colon and the lung in the clinic.

Precision medicine and access to a growing array of therapeutics pose challenges to the diagnostic work-up of patients, which is increasingly met by functional imaging driven by new capabilities in PET/CT instrumentation and AI-based data analysis.
ON102 is an optimized anti-oxMIF antibody with abolished effector functions labeled with 89Zr. It is a radioimmunoconjugate designed for non-invasive PET-imaging applications, including the detection of tumors/metastases or foci of inflammation. This imaging approach provides an opportunity for diagnosis and stratification of patients as well as monitoring treatment for cancer and inflammation.
Currently, OncoOne is developing ON102 in pre-clinical studies for diagnosing and identifying sites of inflammation and cancer.
Current candidates:
ON104 is a second-generation monoclonal anti-oxMIF antibody with abolished effector functions, tailored to the therapeutic needs of patients living with chronic inflammatory and autoimmune disorders. ON104 neutralizes the pleiotropic disease promoting activities of oxMIF. In particular, as oxMIF can override the immunosuppressive effects of glucocorticoids, ON104 synergizes with current immunosuppressant therapeutics.
Currently, OncoOne is advancing ON104 through late-stage pre-clinical development for indications like Rheumatoid Arthritis (RA), Nephritis, Inflammatory Bowel Disease (IBD), and Asthma. Optimized bio-physiochemical properties of ON104 support a high-dose formulation so that patients can perform self-injections at home using an autoinjector pen.

The development of neuroprotective drugs is a significant gap in medical care, as these diseases progressively impact patients' quality of life and ability to perform daily activities. OncoOne is dedicated to addressing these challenges through its proprietary selective targeting approach. By leveraging the presence and pathological activities of oxMIF at sites of chronic inflammation, OncoOne aims to neutralize its disease-promoting activity using bispecific antibodies capable of crossing the blood-brain barrier.
Currently, ON-08 is in the initial discovery stage.
ON203 is a second-generation monoclonal anti-oxMIF antibody designed to address the specific therapeutic requirements of patients with solid tumors. This antibody has been optimized for enhanced tumor penetration and improved pharmacokinetic properties. ON203 effectively inhibits oxMIF-mediated pro-tumorigenic functions and demonstrates cytotoxic functions by inducing antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), offering significant therapeutic potential in solid tumors. Its mechanism of action allows for its use either as a standalone treatment or in combination with standard-of-care therapies.
ON203 is ready for filing IND/IMPD and to address the unmet medical needs of patients facing solid tumors of the colon and the lung in the clinic.

Precision medicine and access to a growing array of therapeutics pose challenges to the diagnostic work-up of patients, which is increasingly met by functional imaging driven by new capabilities in PET/CT instrumentation and AI-based data analysis.
ON102 is an optimized anti-oxMIF antibody with abolished effector functions labeled with 89Zr. It is a radioimmunoconjugate designed for non-invasive PET-imaging applications, including the detection of tumors/metastases or foci of inflammation. This imaging approach provides an opportunity for diagnosis and stratification of patients as well as monitoring treatment for cancer and inflammation.
Currently, OncoOne is developing ON102 in pre-clinical studies for diagnosing and identifying sites of inflammation and cancer.
oxMIF: We Discovered It, We Named It, We Are Drugging It