OncoOne Has Been Pioneering Two Technology Platforms, Anti-oxMIF and Pretarg-It® , Side by Side to Revolutionize Targeted Medicines
Anti-oxMIF Platform
OncoOne takes inspiration from Nobel Laureate Paul Ehrlich’s the “magic bullet” concept and directs modern biomedical research to advance targeted therapeutics for chronic inflammatory and oncological indications.
Our founders have made a groundbreaking discovery: in inflamed tissues and solid tumors the oxidative environment activates the ubiquitously expressed Macrophage Migration Inhibitory Factor (MIF), creating an oxidized MIF isoform, referred to as oxMIF (Thiele, M., Donnelly, S. C. and Mitchell, R. A. (2022)).OxMIF adopts a flexible structure and reveals epitopes usually hidden within the compact MIF trimer. In contrast to its ubiquitous precursor MIF, oxMIF is present only in inflamed or cancerous tissues and thus becomes an invaluable therapeutic target that provides the opportunity to restrict therapeutic activities exclusively to diseased sites.
Focusing on this discovery, we’re developing therapeutic oxMIF-specific antibodies with enormous specificity and selectivity to ensure no interference with the ubiquitous non-pathological MIF.. Besides the therapeutic potential of inhibiting MIF activity in the diseased area, we also explore oxMIF for diagnostic purposes to identify sites of active inflammation and solid tumors.
Our founders have made a groundbreaking discovery: in inflamed tissues and solid tumors the oxidative environment activates the ubiquitously expressed Macrophage Migration Inhibitory Factor (MIF), creating an oxidized MIF isoform, referred to as oxMIF (Thiele, M., Donnelly, S. C. and Mitchell, R. A. (2022)). OxMIF adopts a flexible structure and reveals epitopes usually hidden within the compact MIF trimer. In contrast to its ubiquitous precursor MIF, oxMIF is present only in inflamed or cancerous tissues and thus becomes an invaluable therapeutic target that provides the opportunity to restrict therapeutic activities exclusively to diseased sites.
Focusing on this discovery, we’re developing therapeutic oxMIF-specific antibodies with enormous specificity and selectivity to ensure no interference with the ubiquitous non-pathological MIF. Besides the therapeutic potential of inhibiting MIF activity in the diseased area, we also explore oxMIF for diagnostic purposes to identify sites of active inflammation and solid tumors.
Our founders have made a groundbreaking discovery: in inflamed tissues and solid tumors the oxidative environment activates the ubiquitously expressed Macrophage Migration Inhibitory Factor (MIF), creating an oxidized MIF isoform, referred to as oxMIF (Thiele, M., Donnelly, S. C. and Mitchell, R. A. (2022)). OxMIF adopts a flexible structure and reveals epitopes usually hidden within the compact MIF trimer. In contrast to its ubiquitous precursor MIF, oxMIF is present only in inflamed or cancerous tissues and thus becomes an invaluable therapeutic target that provides the opportunity to restrict therapeutic activities exclusively to diseased sites.
Focusing on this discovery, we’re developing therapeutic oxMIF-specific antibodies with enormous specificity and selectivity to ensure no interference with the ubiquitous non-pathological MIF. Besides the therapeutic potential of inhibiting MIF activity in the diseased area, we also explore oxMIF for diagnostic purposes to identify sites of active inflammation and solid tumors.

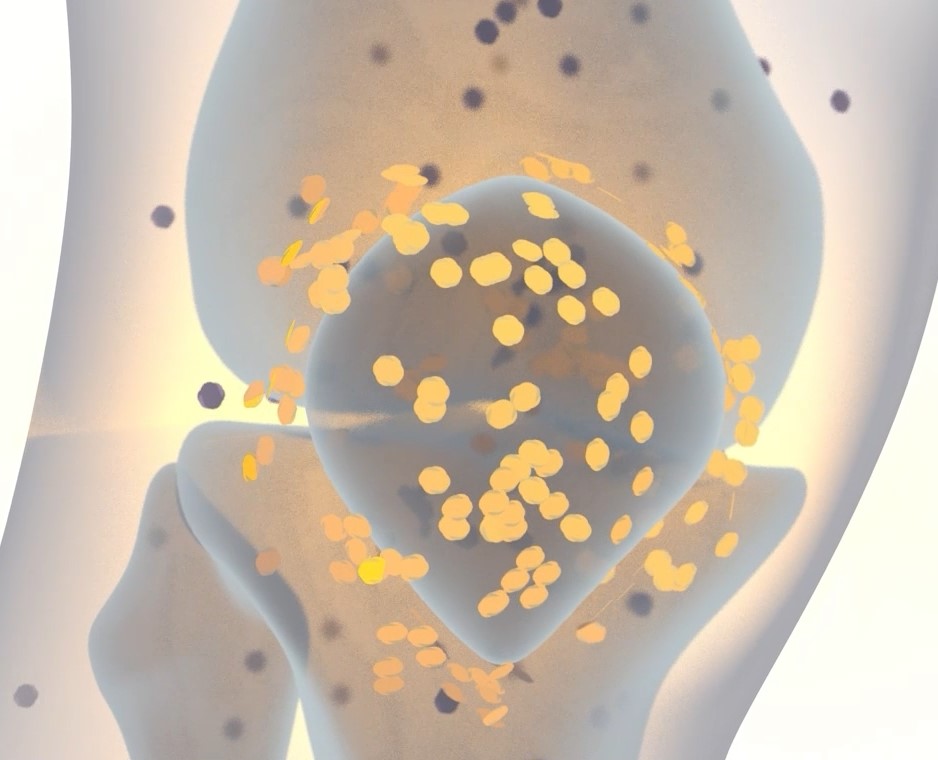
OncoOne’s groundbreaking and versatile approach to direct therapeutics exclusively to diseased sites aims to change therapeutic strategies and enhance patient outcomes in cancer and inflammation.

We leverage the potential of our discovery with a proprietary pipeline of novel antibodies against oxMIF – our highly promising platform of targeted precision medicine, unlocking new therapeutic approaches in immuno-oncology and chronic inflammation.
PreTarg-it® Platform
Leveraging our expertise in antibody engineering, we've created the modular PreTarg-it® platform. It employs bispecific antibodies for pre-targeted radioimmunotherapy, targeting challenging cancers. Our bispecific antibodies ensure optimal tumor penetration and retention, enhancing site-restricted payload delivery.


Our PreTarg-it® Strategy: Ensuring Site-directed Radioactive Delivery


Leveraging our PreTarg-it® platform, we are progressing with four research programs aimed at providing therapeutic solutions for difficult-to-treat solid tumors in the pancreas, ovary, lung, and stomach.

These programs focus on the development of bispecific antibodies that target specific tumor markers such as oxMIF, mesothelin (MSLN), folate receptor-α (FRα), and HER2.